
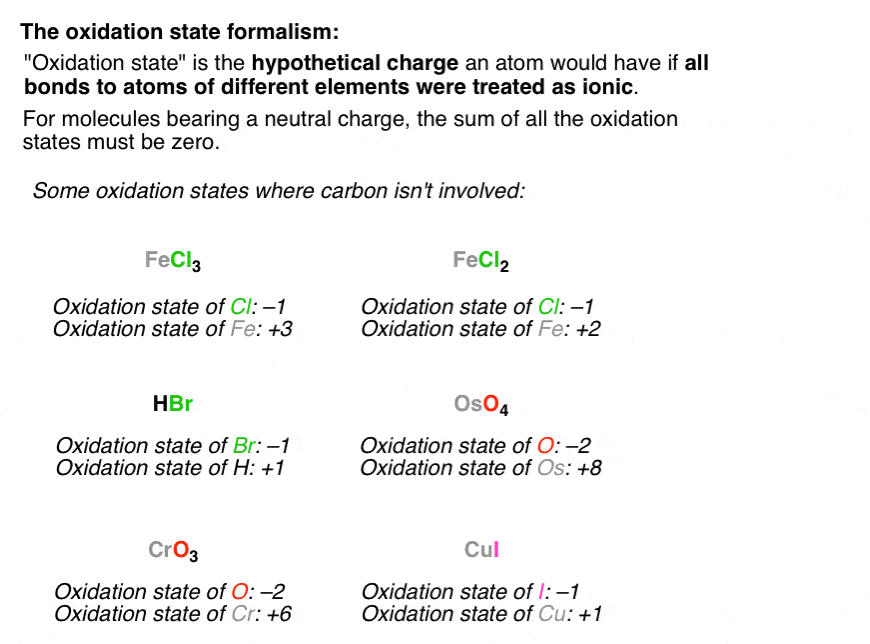


Related:As we know percent yield and theoretical yield can be difficult to calculate. Instead, it only covers the charge over a single atom in the substance. Don't forget that the oxidation state doesn't represent the actual charge over the entire molecule. Thus, the oxidation number or state keeps the record of electrons gained or lost by an atom.Īs we know, the atom which gains electrons develops a negative charge and vice versa the oxidation number could also be negative, positive, and zero as either it loses or gains electrons or stays intact.ĭuring a chemical reaction, we analyze the oxidation state of each element and the corresponding changes in their oxidation states to track the overall electron transfer. In chemistry, we assign each atom a specific number based on the number of electrons it loses or gains during the formation of the compound. What is the oxidation number in chemistry?


 0 kommentar(er)
0 kommentar(er)
